CE Marking
The Nuraleve delivery system features our latest medical device. The CE Marking was granted after verifying the safety and efficacy of our product.
CE Mark No. CE 593638
View License
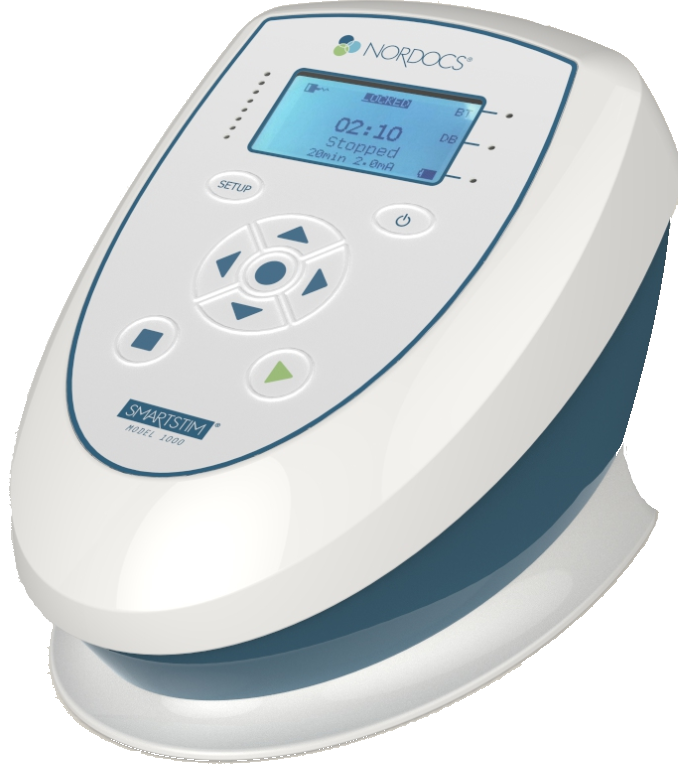
Health Canada Medical Device License
The Nuraleve delivery system features our latest medical device. The medical device license was granted by Health Canada after verifying the safety and efficacy of our product.
Medical Device Licence No. 92093
View License

Health Canada Medical Device Establishment License
Nuraleve is licensed to import and distribute medical devices. The medical device establishment license was issued by Health Canada.
Medical Device Establishment Licence No. 5880
View License

ISO 13485:2016 Certification

 Certified by: BSI Group
Certified by: BSI Group
Certificate #: FS 592661
View Certificate: View Certificate
ISO 13485:2016 Certification (MDSAP)

 Certified by: BSI Group
Certified by: BSI Group
Certificate #: MDSAP 692355
View Certificate: View Certificate
